How Do You Know if Youve Isolated Just One Colony
Overview
Source: Tilde Anderssonane, Rolf Lood1
1 Section of Clinical Sciences Lund, Division of Infection Medicine, Biomedical Center, Lund University, 221 00 Lund, Sweden
Seemingly impossible to determine, microbial biodiversity is truly astounding with an estimated one trillion coexisting species (i,2). Although particularly harsh climates, like the acidic environment of the human being tum (3) or the subglacial lakes of Antarctica (4), may be dominated by a specific species, bacteria are typically found in mixed cultures. As each strain may influence the growth of another (v), the ability to separate and cultivate "pure" (consisting simply of one type) colonies has become essential in clinical and bookish settings alike. Pure cultures enable further genetic (6) and proteomic examinations (seven), assay of sample purity and, peradventure more noteworthy, the identification and characterization of infectious agents from clinical samples.
Bacteria have a wide range of growth requirements and there are numerous types of food media designed to sustain both the un-enervating and the fastidious species (8). Growth media tin can be prepared either in liquid grade (as a broth) or in a typically agar-based (a gelling agent derived from cerise algae) solid grade. Whereas straight inoculation into broth carries the risk of generating a genetically diverse or even mixed bacterial population, plating and re-streaking creates a purer civilization where each cell has a highly like genetic makeup. The streak plate technique is based on progressive dilution of a sample (Figure 1), with the aim of separating individual cells from one some other. Any viable prison cell (futurity referred to as a colony forming unit, CFU) sustained by the media and designated surroundings can afterwards found an isolated colony of daughter-cells through binary fission. In spite of the rapid mutation rates within bacterial communities, this cell-grouping is generally regarded as clonal. Harvesting and re-streaking this population consequently ensures that subsequent work involves but a single bacterial type.
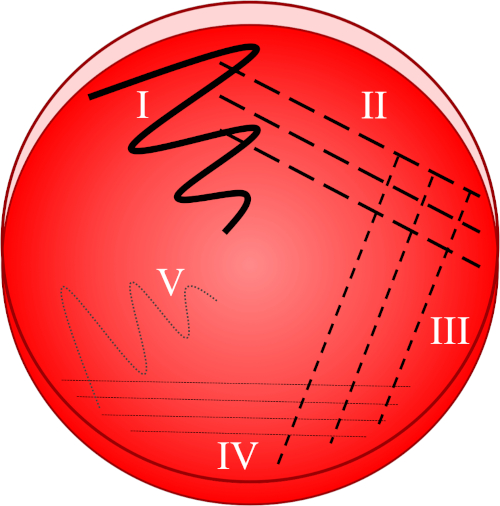
Effigy one: A streak plate is based on progressive dilution of the original sample. I) The inoculum is initially dispersed using a zig-zag motion, creating an area with a relatively dense bacterial population. II-IV) Streaks are drawn from the preceding expanse, using a sterile inoculation loop each fourth dimension, until the fourth quadrant is reached. V) A terminal zig-zag motion directed towards the middle of the plate forms a region where the inoculum has been markedly diluted, assuasive colonies to appear split up from one another.
The streak plate technique can also exist combined with the use of selective and/or differential media. A selective medium will inhibit the growth of certain organisms (e.m. through addition of antibiotics) whilst a differential medium will solely help distinguish one from another (e.g. through hemolysis on blood agar plates).
Underlying all work in microbiology is the use of hygienic (sterile) techniques. Every bacterial culture should be considered potentially pathogenic as in that location is a risk of unintended growth of treacherous strains, aerosol formation and contagion of equipment/personnel. To minimize these risks, all media, plastic-, metal- and drinking glass-ware are typically sterilized through autoclaving earlier and later on use, subjecting them to loftier-pressure saturated steam at around 121°C that effectively wipes out any lingering cells. The work space is generally disinfected using ethanol both prior to, and after, use. Lab coat and gloves are e'er worn during piece of work with infectious agents.
Procedure
Log in or Start trial to access full content. Learn more about your institution's access to JoVE content here
1. Set up
- All microbes should be treated as if they are hazardous. E'er wearable a lab coat and gloves, tie dorsum long hair, and ensure that whatsoever wounds are particularly well protected.
- Ready the work infinite by sterilizing it using 70% ethanol.
- Ensure that agar plates, sample solution(s) and either a box of pre-sterilized plastic inoculation loops or a metallic loop plus a Bunsen flame, are close at paw. Dispensable, plastic loops are typically pre-sterilized. Metal loops should be dipped in 70% ethanol, and then held near the blue expanse of a Bunsen flame and heated until fiery hot. Allow the wire to cool down by raising the lid of the plate (only slightly to foreclose contamination) and tapping it against the solidified medium.
- Finish each procedure with a repeated sterilization of the work space and a thorough launder/sterilization of easily and wrists.
2. Protocol
- Preparation of media
- Identify and prepare a solid medium (typically containing 1.v% (w/v) agar) that volition sustain the utilized bacterial species/strain. Mix the medium in a bottle able to agree twice the final volume to avoid overflow when autoclaving.
- Sterilize the media by placing the bottle, with a semi-tightened cap, in an autoclave set to 121°C for 20 min.
- Close the cap properly as soon as the canteen is removed from the autoclave. If the media is to exist used shortly, place the bottle in a water-bath set to 45°C to preserve it in a liquid state. The agar will otherwise solidify at anything less than 32-xl°C, and can later exist re-heated (typically using a microwave) to a melting point at 85°C.
- Preparation of culture plate(s)
- Mark the base of sterile Petri dishes (typically 100 x fifteen mm) on either the side or the bottom with the experimenter'due south proper name, the date, and media blazon.
- Pour twenty-25 mL of 45°C agar culture medium (previously prepared) into each of the labelled plates. Should cream appear along the edges, this should be swiftly removed using a regular pipette and a sterile tip.
- Immediately identify all lids dorsum onto the dishes to preclude contamination.
- Permit the agar to solidify for approximately 2 h at room temperature or overnight at 4°C. Once set, bacterial civilization plates should afterwards exist stored upside-down at four°C to minimize condensation on the medium surface.
- Streak plating
- Submerge a sterile loop into the desired inoculum and immediately disperse the collected sample onto the first quadrant of the plate using a zig-zag motion (Figure i, I).
- Close the lid and re-sterilize the inoculation loop or collect a new sterile dispensable loop.
- Brand 3-4 strokes radiating from the start quadrant (containing a relatively dumbo bacterial population) towards the second quadrant of the plate (Figure 1, 2).
- Shut the chapeau and re-sterilize the inoculation loop or discard the dispensable loop and collect a new sterile 1.
- Repeat this streaking of three-iv strokes from the second into the third quadrant, and then from the 3rd to the fourth quadrant, using a sterile loop each time (Figure 1, 3 - Four).
- Using a sterile loop, make 1 final stroke in a zig-zag pattern from the fourth quadrant towards the middle of the plate (Effigy 1, V). The bacterial prevalence will be lower in this area, ideally allowing private colonies to exist established from a unmarried, viable mother-cell.
- Close the lid and (if required by the bacterial species) seal with parafilm to prevent air menstruation.
- Depending on the bacterial species/strain, place the culture plate up-side-down in a suitable environment and incubate until bacterial colonies are visible (segregated colonies can announced in either area of the plate as the initial concentration may vary).
- To generate a clonal bacterial population, streak out some other plate, exchanging the inoculum plated onto the original plate for cells isolated from a unmarried colony of the original plate.
On a Petri dish, if a unmarried bacterium undergoes multiple rounds of asexual reproduction, it will atomic number 82 to the formation of a clonal colony. However, obtaining a single bacterium from a mixed sample, such as a soil pause, can be difficult. If one loopful of this heterogeneous civilisation is taken, it tin contain as many as i trillion individual bacteria. To spread this many leaner out onto the surface of an agar plate and obtain a unmarried colony, even using a zig-zag pattern, the loop would demand to be dragged continuously over the surface of enough plates set side-past-side to encircle the entirety of Liberty Isle. Obviously, scientists do not really utilize that many plates. Instead, they use a technique called streak plating.
The streak plate technique is based on progressive dilution of a bacterial sample, and information technology is performed over the solid media surface of a single Petri dish. To begin, the media surface is visually divided into five sections past assigning four fragments of the circumference as the start iv sections, and the plate's center as the fifth. This will effectively create five media plates out of a single Petri dish. Next, using a loopful of desired inoculum, the start department is streaked using a zig-zag design. And so, either a new disposable loop is used, or in the case of a wire loop, it is sterilized with a Bunsen burner, flaming it until it is red hot along the length of the wire. This employ of a new loop, or flame sterilize loop, removes any remaining bacterial cells, assisting in the dilution of the bacteria. The hot loop is and then cooled in the air for a few seconds before existence dragged through the first section to create iii to four separate lines, each conveying only a fraction of leaner into the 2nd section. The remaining sections are streaked in the same manner, using a sterile loop each time, and a single pass through the previous streak.
Using this wheel of streaking and sterilizing, the bacterial concentration in every subsequent department should be diluted then that the last section contains only a few discretely located bacteria. Upon incubation, these detached leaner multiply to produce isolated clonal colonies of girl cells, which are referred to every bit Colony Forming Units, or CFUs. These can exist harvested and re-streaked to ensure that subsequent work involves only a single bacterial type, referred to as a pure civilization. Every bit well every bit isolating unmarried colonies from a mixed-bacterial civilization, the streak plating technique is also used to select media-specific strains, decide bacterial colony morphology, or identify different bacterial species. In this video, nosotros will demonstrate how to isolate single-bacterial colonies from a mixed-bacterial sample suspension via streak plating technique.
To begin, put on laboratory gloves and a lab coat. Next, sterilize the workspace using 70% ethanol. Next, select a suitable medium that will sustain the utilized bacterial species or strain and begin preparing the media. Here, common LB agar is prepared past weighing out 10 grams of pre-formulated, powdered media and 7.five grams of agar. Add together the weighed, stale components to a glass bottle which is able to concord twice the last book to avoid overflow. Then, add 500 milliliters of water to the bottle, and cap it semi-tightly. Sterilize the media past placing the bottle in an autoclave set to 121 degrees Celsius for twenty minutes. After completion, utilize heat-proof gloves or a hot pad to remove the media from the machine then immediately twist the canteen cap to close it tightly.
For the aforementioned-mean solar day use, allow the media cool down past placing the bottle into a water bathroom heated to approximately 45 degrees Celsius, to preserve the media in a liquid state. Alternatively, the media tin can be left at room temperature to store at solid state. When needed, microwave the canteen with the lid slightly open to melt the media, and permit the media to cool using a 45 degree Celsius h2o bath.
Side by side, take a sleeve of sterile Petri dishes, and with a permanent marker, characterization them with the investigator and media names besides equally the date. Then, transfer the required volume of media into a sterile vessel, and add antibiotics or other sensitive components if necessary. Here, fifty milliliters of media is mixed with 100 microliters of Kanamycin for a final concentration of 25 micrograms per milliliter. Swirl the tube to ensure even distribution of the added components throughout the media. Slowly, so as to avoid bubble germination, cascade 20 to 25 milliliters of approximately 45 degree Celsius civilisation medium into each of the plates. If bubbles or foam appear, swiftly remove using a regular pipette and a sterile tip. And then, immediately replace all lids to prevent contamination. Permit the agar to solidify at room temperature for at least two hours or overnight. Once solidified, store the civilization plates upside down at four degrees Celsius to minimize condensation on the medium's surface.
To streak the civilization of choice, first take a clean culture plate and remove the lid. Working quickly, submerge a disposable, sterile loop into the desired inoculum and and then immediately swab the loop over the first quadrant of the plate using a zig-zag motion. Supplant the lid of the dish, discard the used inoculation loop, and and so select a new sterile loop. Using the new loop, make three to four strokes crossing the original swab line radiating from the first quadrant, which should contain a relatively dense leaner population into the 2nd quadrant. Close the chapeau once more, and discard the loop. With a new loop, echo this action over again, simply this time streaking from the 2nd into the third quadrant. Then, with a new loop again, make some other streak from the third into the fourth section of the plate. Finally, with a fresh loop, make one last stroke in a zig-zag pattern from the 4th quadrant towards the eye of the plate. The bacterial prevalence will be lower in this area, ideally allowing individual colonies to be established from a unmarried viable mother cell.
Replace the plate lid, and if appropriate for the bacterial species, seal the plate with para moving picture to forbid airflow. Turn the culture plate upside down to prevent condensation drips, and so identify at a suitable temperature for growth. Hither, an incubator is set to 37 degrees Celsius. Let the plate to incubate until bacterial colonies are visible. To generate a clonal bacterial population, select one discrete colony from this plate. Now, with the sterile loop, touch the target colony, and as before, make a streak in the starting time quadrant of a new plate. Go along to alternately sterilize the loop and streak the remaining quadrants of the plate as previously demonstrated, catastrophe with the zig-zag to the middle. Close the plate, and place it to incubate until discrete colonies course. Once these colonies are grown, they will typically correspond pure clonal strains.
The initial streak plate may contain colonies originating from cells from dissimilar bacterial species or cells with dissimilar genetic makeup, depending upon the sample purity. Through subsequent isolation of a single colony, where all units are derived from a common mother cell, the second streaking process generates a relatively clonal bacterial population, suitable for further characterization or inoculation into broth.
Subscription Required. Delight recommend JoVE to your librarian.
Results
Log in or Get-go trial to access full content. Larn more nearly your institution's admission to JoVE content here
The initial streak-plate may contain colonies originating from cells with different genetic makeup or (depending on sample purity) from different bacterial species (Effigy ii A).
Through subsequent isolation of a single colony, where all units are derived from a common female parent-cell, the second streaking procedure generates a relatively clonal bacterial population, suitable for further characterization or inoculation into broth (Figure 2 B).

Effigy 2: A pure culture can be generated from a mixed sample through isolation of a single, secluded colony. A) Growth of a single bacterial jail cell (CFU) generated a clonal colony, separated from those of other species and strains. This CFU was used for subsequent streaking onto a new plate B) A second plate, where the bacterial population consists solely of cells derived from the initial CFU.
Subscription Required. Please recommend JoVE to your librarian.
Applications and Summary
Log in or Showtime trial to access full content. Acquire more about your institution'south admission to JoVE content here
The ability to obtain and cultivate a pure bacterial colony is essential, both in clinical and academic settings. Streak plating enables the isolation of a relatively clonal cell population, originating from a shared CFU, that may be of particular interest during diagnosis or for additional label of the isolate. A sample is streaked onto a suitable agar-based nutrient medium and incubated until colonies become visible. An isolated colony is after harvested and re-streaked onto a second plate.
Subscription Required. Please recommend JoVE to your librarian.
References
- The Human Microbiome Project C. Construction, Function and Diversity of the Healthy Human Microbiome. Nature. 486:207-214. (2012)
- Locey KJ, Lennon JT. Scaling laws predict global microbial diversity. Proceedings of the National Academy of Sciences. 113 (21) 5970-5975 (2016)
- Skouloubris S, Thiberge JM, Labigne A, De Reuse H. The Helicobacter pylori UreI protein is not involved in urease action simply is essential for bacterial survival in vivo. Infection and Immunity. 66:4517-21. (1998)
- Mikucki JA, Auken Due east, Tulaczyk Southward, Virginia RA, Schamper C, Sørensen KI, Doran PT, Dugan H, Foley N. Deep groundwater and potential subsurface habitats beneath an Antarctic dry valley. Nature Communications. 6:6831. (2015)
- Mullineaux-Sanders C, Suez J, Elinav East, Frankel G. Sieving through gut models of colonization resistance. Nature Microbiology. three:132-140. (2018)
- Fournier PE, Drancourt Thou, Raoult D. Bacterial genome sequencing and its use in infectious diseases. Lancet Infectious Diseases. vii:711-23 (2007)
- Yao Z, Li Westward, Lin Y, Wu Q, Yu F, Lin W, Lin X. Proteomic Assay Reveals That Metabolic Flows Affect the Susceptibility of Aeromonas hydrophila to Antibiotics. Scientific Reports. 6:39413 (2016)
- Medina D, Walke JB, Gajewski Z, Becker MH, Swartwout MC, Belden LK. Civilization Media and Private Hosts Bear upon the Recovery of Culturable Bacterial Diversity from Amphibian Pare. Frontiers in Microbiology. viii:1574 (2017)
On a Petri dish, if a unmarried bacterium undergoes multiple rounds of asexual reproduction, it will pb to the formation of a clonal colony. However, obtaining a single bacterium from a mixed sample, such as a soil suspension, tin can be hard. If one loopful of this heterogeneous culture is taken, it can contain equally many as one trillion individual leaner. To spread this many bacteria out onto the surface of an agar plate and obtain a unmarried colony, even using a zig-zag pattern, the loop would need to be dragged continuously over the surface of enough plates set side-by-side to encircle the entirety of Freedom Island. Evidently, scientists do not really use that many plates. Instead, they use a technique called streak plating.
The streak plate technique is based on progressive dilution of a bacterial sample, and it is performed over the solid media surface of a single Petri dish. To brainstorm, the media surface is visually divided into five sections by assigning four fragments of the circumference as the offset four sections, and the plate'due south center equally the fifth. This will effectively create five media plates out of a single Petri dish. Next, using a loopful of desired inoculum, the get-go department is streaked using a zig-zag pattern. Then, either a new disposable loop is used, or in the case of a wire loop, it is sterilized with a Bunsen burner, flaming information technology until it is red hot along the length of the wire. This use of a new loop, or flame sterilize loop, removes any remaining bacterial cells, profitable in the dilution of the bacteria. The hot loop is then cooled in the air for a few seconds before being dragged through the first section to create three to four separate lines, each carrying only a fraction of leaner into the 2nd section. The remaining sections are streaked in the aforementioned way, using a sterile loop each time, and a single pass through the previous streak.
Using this cycle of streaking and sterilizing, the bacterial concentration in every subsequent section should be diluted so that the concluding section contains but a few discretely located bacteria. Upon incubation, these discrete bacteria multiply to produce isolated clonal colonies of daughter cells, which are referred to every bit Colony Forming Units, or CFUs. These can be harvested and re-streaked to ensure that subsequent work involves merely a single bacterial blazon, referred to as a pure civilization. As well as isolating single colonies from a mixed-bacterial civilisation, the streak plating technique is also used to select media-specific strains, decide bacterial colony morphology, or identify dissimilar bacterial species. In this video, we will demonstrate how to isolate unmarried-bacterial colonies from a mixed-bacterial sample suspension via streak plating technique.
To begin, put on laboratory gloves and a lab coat. Next, sterilize the workspace using 70% ethanol. Next, select a suitable medium that will sustain the utilized bacterial species or strain and begin preparing the media. Here, common LB agar is prepared by weighing out ten grams of pre-formulated, powdered media and 7.v grams of agar. Add the weighed, dried components to a glass bottle which is able to hold twice the final volume to avoid overflow. Then, add 500 milliliters of h2o to the bottle, and cap information technology semi-tightly. Sterilize the media by placing the bottle in an autoclave fix to 121 degrees Celsius for twenty minutes. After completion, employ rut-proof gloves or a hot pad to remove the media from the automobile then immediately twist the canteen cap to shut it tightly.
For the same-mean solar day utilize, allow the media cool downward past placing the bottle into a water bath heated to approximately 45 degrees Celsius, to preserve the media in a liquid state. Alternatively, the media tin exist left at room temperature to store at solid state. When needed, microwave the bottle with the lid slightly open to melt the media, and allow the media to cool using a 45 caste Celsius water bath.
Adjacent, take a sleeve of sterile Petri dishes, and with a permanent marking, characterization them with the investigator and media names likewise as the engagement. And then, transfer the required volume of media into a sterile vessel, and add together antibiotics or other sensitive components if necessary. Here, l milliliters of media is mixed with 100 microliters of Kanamycin for a concluding concentration of 25 micrograms per milliliter. Swirl the tube to ensure even distribution of the added components throughout the media. Slowly, and so as to avoid chimera formation, pour 20 to 25 milliliters of approximately 45 degree Celsius civilisation medium into each of the plates. If bubbles or cream appear, swiftly remove using a regular pipette and a sterile tip. Then, immediately replace all lids to forestall contagion. Permit the agar to solidify at room temperature for at least two hours or overnight. Once solidified, store the civilisation plates upside down at four degrees Celsius to minimize condensation on the medium's surface.
To streak the civilization of choice, showtime accept a clean culture plate and remove the lid. Working quickly, submerge a dispensable, sterile loop into the desired inoculum and and then immediately swab the loop over the first quadrant of the plate using a zig-zag motion. Replace the chapeau of the dish, discard the used inoculation loop, and so select a new sterile loop. Using the new loop, make three to 4 strokes crossing the original swab line radiating from the first quadrant, which should contain a relatively dense bacteria population into the second quadrant. Close the lid once more, and discard the loop. With a new loop, repeat this action again, but this time streaking from the second into the tertiary quadrant. Then, with a new loop again, make another streak from the third into the fourth section of the plate. Finally, with a fresh loop, make one concluding stroke in a zig-zag blueprint from the fourth quadrant towards the center of the plate. The bacterial prevalence will be lower in this area, ideally assuasive individual colonies to be established from a single viable female parent cell.
Replace the plate lid, and if appropriate for the bacterial species, seal the plate with para movie to prevent airflow. Plough the culture plate upside down to prevent condensation drips, and and so place at a suitable temperature for growth. Hither, an incubator is set up to 37 degrees Celsius. Let the plate to incubate until bacterial colonies are visible. To generate a clonal bacterial population, select one discrete colony from this plate. At present, with the sterile loop, touch the target colony, and as before, make a streak in the first quadrant of a new plate. Proceed to alternately sterilize the loop and streak the remaining quadrants of the plate as previously demonstrated, ending with the zig-zag to the center. Close the plate, and identify it to incubate until discrete colonies form. In one case these colonies are grown, they will typically represent pure clonal strains.
The initial streak plate may incorporate colonies originating from cells from different bacterial species or cells with dissimilar genetic makeup, depending upon the sample purity. Through subsequent isolation of a single colony, where all units are derived from a common mother cell, the second streaking procedure generates a relatively clonal bacterial population, suitable for further label or inoculation into broth.
Source: https://www.jove.com/v/10509/pure-cultures-streak-plating-isolation-single-bacterial-colonies-from
0 Response to "How Do You Know if Youve Isolated Just One Colony"
Post a Comment